Femara
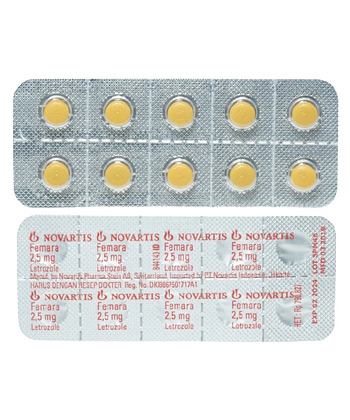
Femara
- In our pharmacy, you can buy Femara without a prescription, with delivery in 5–14 days throughout Canada (English). Discreet and anonymous packaging.
- Femara is intended for the treatment of hormone receptor-positive breast cancer in postmenopausal women. The drug works as an aromatase inhibitor, reducing estrogen levels to slow or stop the growth of certain types of breast tumors.
- The usual dose of Femara is 2.5 mg once daily.
- The form of administration is a tablet.
- The effect of the medication begins within a few days, but optimal effectiveness may take several weeks.
- The duration of action is approximately 24 hours.
- Do not consume alcohol.
- The most common side effect is hot flushes.
- Would you like to try Femara without a prescription?
Basic Femara Information
- INN (International Nonproprietary Name): Letrozole
- Brand names available in Canada (English): Femara
- ATC Code: L02BG04
- Forms & dosages (e.g., tablets, injections, creams): Oral film-coated tablets, 2.5 mg
- Manufacturers in Canada (English): Novartis Pharma AG
- Registration status in Canada (English): Approved
- OTC / Rx classification: Prescription Only (Rx)
Availability & Price Landscape
Femara (Letrozole) is widely accessible across Canada, available at major pharmacy chains such as Shoppers Drug Mart, Rexall, and London Drugs.
The medication typically comes in packaging sizes of 2.5 mg tablets, usually in blister packs. Pricing can differ based on the province, reflecting the various provincial health plans and drug coverage programs like the Ontario Drug Benefit or BC PharmaCare.
Online Pharmacy Trends In Canada
The trend of purchasing Femara online has seen significant growth in Canada, as patients seek convenience and potentially lower prices.
However, regulations restrict certain pharmacies from operating across provincial boundaries. Many Canadians are now comparing prices between licensed online pharmacies and local retail options, often finding cost advantages when factoring in shipping and handling fees.
Canadian Patient Insights & Satisfaction Levels
Online platforms like Reddit Canada, HealthBoards, and AskDocs have become popular venues for Canadian patients to share their experiences with Femara.
These forums provide essential insights into the medication's effectiveness, side effects, and various personal anecdotes, supporting potential users in making informed decisions about their treatment options.
Reported Benefits And Challenges From Canadian Patients
Patients often look to express the benefits they’ve experienced with Femara, such as improved cancer outcomes, which contribute to greater hope in their treatment journey.
Despite this, many also voice challenges, including common side effects like hot flushes and fatigue. This dialogue within community threads often reflects a blend of optimism about treatment plans integrating Femara, along with valid concerns over its adverse effects.
Product Overview & Brand Variants
In Canada, Femara is recognized by its International Nonproprietary Name (INN), Letrozole, and is primarily marketed under the Femara brand.
While other international alternatives include Letros or Letrozol, Femara remains the predominant choice in Canadian healthcare for hormone receptor-positive breast cancer management.
Legal Classification Under Health Canada
Femara is classified as a prescription-only medication in Canada. This classification mandates that a valid prescription is required from a licensed healthcare provider, ensuring strict adherence to healthcare standards.
The approval process by Health Canada guarantees that the medication meets essential safety and efficacy standards necessary for treating hormone receptor-positive breast cancer.
Indications In Local Canadian Medical Practice
Health Canada has approved Femara primarily for treating hormone receptor-positive breast cancer in postmenopausal women. This indication is highlighted by its Drug Identification Number (DIN) and reflects the medication's therapeutic focus.
Although its primary use is for cancer treatment, off-label discussions around Letrozole have emerged in Canadian healthcare settings concerning fertility issues. Some healthcare providers may prescribe Femara for ovulation induction, but this off-label usage is not officially sanctioned and requires careful patient monitoring.
How It Works In The Body
Letrozole functions by reducing the amount of estrogen produced within the body. In the context of hormone-sensitive cancers, this decrease can significantly hinder the growth of cancer cells.
Patients often find it beneficial to understand that the medication's effectiveness in controlling cancer progression ties closely to its ability to lower estrogen levels.
Clinical Detail From Health Canada Resources
From a clinical standpoint, Letrozole specifically targets aromatase, an enzyme pivotal in transforming androgens into estrogens. This inhibition effectively lowers estrogen levels, disrupting growth signals present in estrogen receptor-positive breast cancer tissues, as highlighted in resources provided by Health Canada.
Dosage & Administration
When it comes to Femara, understanding the dosage and administration is crucial to get the most out of treatment. Typically, Femara 2.5 mg is taken once daily, easily fitting into a routine with or without meals. The recommended duration for treatment can extend from 5 to 10 years, depending on clinical guidelines for adjuvant therapy. These guidelines in Canada align closely with those of other countries, ensuring standardised care and efficiency in breast cancer treatments.
Standard regimens per Canadian guidelines
Femara is primarily prescribed for individuals with hormone-receptor-positive breast cancer, often as an adjuvant therapy following surgery. This regimen is based on extensive research, indicating that patients benefit considerably from prolonged treatment up to a decade. Such durations help in reducing the recurrence of cancer significantly, thereby enhancing survival rates. Following professionals' directions and adhering to prescribed dosages can be essential in achieving optimal outcomes.
Adjustments by patient type
In the Canadian clinical landscape, adjustments to Femara dosages are generally not necessary for elderly patients or those with mild to moderate liver or renal impairment. The focus is on monitoring the patient’s response throughout the treatment journey. Physicians might tailor the dosage based on individual tolerance levels and side effects experienced, ensuring that the therapeutic benefits are maximised while minimising discomfort.
Contraindications & Side Effects
Like all medications, Femara comes with its share of potential side effects that patients need to be aware of. Recognising these is a key part of the treatment plan and overall cancer care strategy.
Common
Among the common side effects reported by patients using Femara include hot flushes, musculoskeletal pain, fatigue, and nausea. These experiences can vary significantly in intensity. Many individuals seek support groups to share their journeys and coping strategies, enhancing their emotional wellbeing. Health Canada keeps a close watch on these reported side effects through robust pharmacovigilance systems.
Rare but serious
Although rare, serious side effects can include severe allergic reactions or significant bone density loss, which is particularly concerning for long-term users. Data gathered within the Canadian healthcare system indicate that patients experiencing unusual symptoms should immediately consult their healthcare provider. Adjusted treatment plans may become necessary to address emerging health concerns.
Comparable Medicines in Canada
When exploring Femara, it is also important to understand other comparable medications available in Canada. Patients should have a clear view of their options to make informed decisions.
Alternatives table
| Medication | Type | DIN |
|---|---|---|
| Anastrozole | Non-steroidal aromatase inhibitor | [Insert DIN] |
| Exemestane | Steroidal aromatase inactivator | [Insert DIN] |
| Tamoxifen | Selective Estrogen Receptor Modulator | [Insert DIN] |
Pros and cons list
A closer look at Femara reveals several advantages and disadvantages for patients to consider:
- Pros: Effective in hormone-receptor-positive cases and comes with the convenience of once-daily dosing.
- Cons: Common side effects may dissuade some patients; ongoing monitoring is essential throughout treatment.
Current Research & Trends
The landscape surrounding Femara is dynamic, with ongoing research illuminating its role in breast cancer treatment.
Major Canadian or international studies 2022–2025
Numerous clinical trials currently underway across Canada and globally are evaluating the optimal duration of Femara usage and its outcomes in various stages of breast cancer. These studies strive to refine treatment protocols while ensuring enhanced patient support and understanding.
Emerging trends in Femara usage
New evidence suggests that letrozole, the active ingredient in Femara, might offer advantages even in early-stage treatment scenarios. Research is increasingly focusing on its long-term efficacy and the profiles of side effects experienced by patients, often discussed in supportive community forums.
Common Patient Questions in Canada
Patients frequently have several queries regarding Femara, particularly as they navigate their treatment journey.
Patient queries
Some common questions centre around:
- What side effects to expect while on Femara?
- How should missed doses be managed?
- Can Femara be used for fertility issues?
Addressing these concerns in a straightforward, patient-friendly manner serves to enhance overall understanding and boosts adherence to treatment plans. Sharing real-life experiences can also foster a supportive environment for patients undergoing similar journeys with Femara.
Regulatory Status
The approval of Femara through Health Canada involved a meticulous evaluation process that focused on its efficacy and safety for the populations it aims to treat. This rigorous process ensures that approved medications meet high-quality standards, fostering trust among consumers and healthcare providers alike. Femara has been assigned a Drug Identification Number (DIN), allowing for straightforward identification of approved medications in Canada. This system is crucial in monitoring the products available in the market.
Health Canada approval process
Femara’s journey through Health Canada’s approval process highlights the stringent checks put in place for drug safety. The assessment focuses on various elements, including clinical trials that demonstrate the drug's effectiveness in treating specific conditions, particularly hormone receptor-positive breast cancer in postmenopausal women.
DIN number relevance
The DIN assigned to Femara is not just a mere label; it signifies regulatory approval and details the formulation used within the Canadian market. This number equips healthcare providers with essential information for prescribing, ensuring treatments align with set guidelines, and enhances safety in medication management.
Visual Recommendations
Engaging visual representations can greatly assist patients in understanding Femara's usage and effects. Infographic ideas could include side effects, recommended dosage schedules, and comparisons with alternative treatments, tailored specifically for Canadian patients. These visual aids can be used in clinic environments or online, effectively enhancing patient education and informed decision-making.
Infographic ideas for Canadian context
Visual aids that clarify common side effects associated with Femara, dosage guidelines, and treatment comparisons can empower patients on their treatment paths. Infographics showing statistics on treatment success rates and potential side effects provide quick reference points for patients, helping them make informed choices amidst their treatment journeys.
Suggested visual aids for patient engagement
Interactive resources designed for patients might include tools that help track medication schedules or visual timelines detailing treatment regimens. These can illustrate the benefits of long-term use and expectations on side effects, fostering an environment where patients feel more in control of their treatment plans.
Buying & Storage Advice
Buying Femara involves considerations for safety and convenience, whether through in-store or online options. While in-store pharmacies may offer immediate consultations with pharmacists, online options can provide cost savings. It's vital for online consumers to verify that these pharmacies are licensed to operate in Canada, ensuring compliance with safety standards.
In-store vs. online Canadian purchase tips
Opting for in-store purchases provides the benefit of direct interaction with pharmacists who can answer questions and guide proper use of Femara. On the other hand, online pharmacies might offer competitive prices. Always ensure these online platforms are Government-approved to guarantee safe practices are upheld.
Proper storage with Canadian climate considerations
Femara should be stored below 30°C to maintain its integrity and efficacy. In Canada’s variable climate, it’s vital to keep the medication away from moisture and heat. Particularly during transportation, storage conditions should be monitored to ensure that the drug remains effective and safe for consumption.
Guidelines for Proper Use
When using Femara, adherence to dosage guidelines provided by Canadian healthcare providers is essential. Regular assessments of patient progress by doctors and pharmacists enable optimal management of the treatment plan, helping identify any side effects and adjust doses as necessary for better outcomes.
Canadian doctor/pharmacist advice style
Healthcare providers routinely stress the importance of sticking to the prescribed doses of Femara. Patients should be educated not only on when and how to take the medication, but also on how to manage potential side effects, ensuring a comprehensive approach to treatment.
Patient resources for ongoing support
Various resources are available for patients, including community support networks and medication management tools that help them track their treatments. Maintaining regular follow-ups with healthcare providers supports ongoing evaluation and necessary adjustments in treatment, reinforcing the awareness of the medication's impact.
| City | Region | Delivery time |
|---|---|---|
| Toronto | Ontario | 5–7 days |
| Vancouver | British Columbia | 5–7 days |
| Montreal | Quebec | 5–7 days |
| Calgary | Alberta | 5–7 days |
| Ottawa | Ontario | 5–7 days |
| Edmonton | Alberta | 5–7 days |
| Winnipeg | Manitoba | 5–7 days |
| Halifax | Nova Scotia | 5–9 days |
| Victoria | British Columbia | 5–9 days |
| Regina | Saskatchewan | 5–9 days |
| Saskatoon | Saskatchewan | 5–9 days |
| St. John's | Newfoundland | 5–9 days |
| London | Ontario | 5–9 days |
| Kitchener | Ontario | 5–9 days |